Ubuwazi ngokusabela okungcwele komakhemikhali?
2024,04,22
Uma kukhulunywa ngogesi wemvelo, akumele ungajwayelani ngakho, futhi akunazinsuku abukho umndeni ongapheka ngaphandle kwawo. Ingxenye eyinhloko yegesi yemvelo yi-methane, eyinye yezinhlanganisela ze-hydrocarbon elula. Ukusheshisa ukuthuthukiswa kanye nokusetshenziswa kwe-methane kuyisihluthulelo sokuthola ukuthuthukiswa okuluhlaza nokuzinzile kwemboni yamakhemikhali. Ngaphezu kokusetshenziswa kwayo okuqondile njengophethiloli, i-methane nayo ingasetshenziswa njengomthombo we-C1, okungukuthi, i-molecule equkethe i-athomu yekhabhoni futhi iqhubeke nokuguqulwa ukuze kulungiswe amakhemikhali angezwe angezwe aphezulu, njenge-methanol, i-formic i-acid nokunye. IMethane ingashiswa ngo-O oxygen ukwakha amanzi kanye nekhabhoni dioksijini. Ngaphandle kokuvutha, kungenzeka yini ukwenza kusebenze futhi uguqule amabhodlela e-hydrocarbon ama-molekyuli we-methane ngaphansi kwezimo ezimnene? Impendulo inguyebo! Le "phoyisa elingcwele" ukusabela emkhakheni weCatalysis. Ukusabela okuhlotshaniswa ne- "Holy Grail" kuvame ukuba yinselele enkulu, ngoba kungadingeka ukuthi kwenziwe ngaphansi kwezimo ezinzima kakhulu, noma kungadingeka ukunqoba ubunzima obukhona bokusabela kwamakhemikhali, njengokusebenzisa izinhlanganisela ezizinzile kakhulu, eziphansi izithelo, nokukhetha okuphansi. Lezi zinselelo zenza kube nzima ukuqaphela lokhu kusabela, kepha uma zingatholakala ngempumelelo, zizoholela ekuqhekekeni okukhulu ocwaningweni lwesayensi kanye nezinhlelo zezimboni.

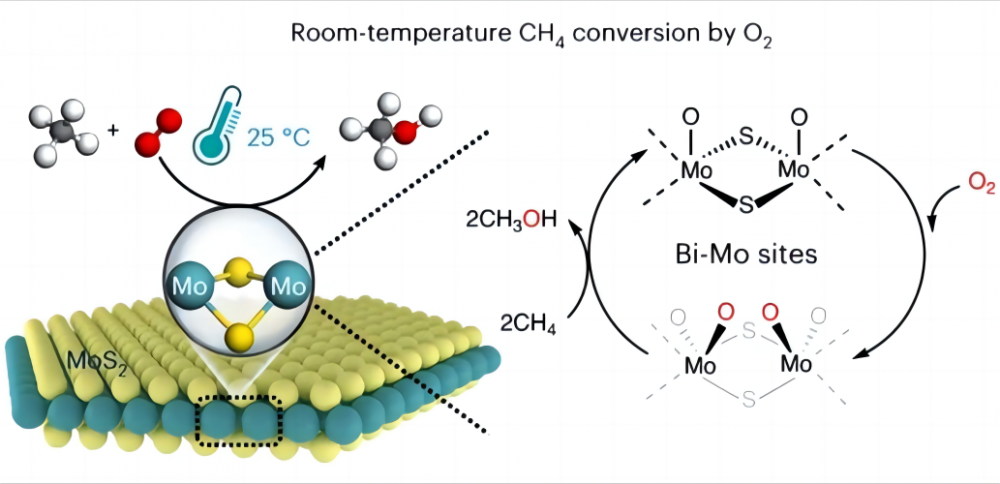
1.Challenges ekuguqulweni kwe-methane emazingeni okushisa aphansi Kunzima kakhulu ukuguqula i-methane ngqo kwamanye amakhemikhali awusizo nge-oksijini eshibhile emazingeni okushisa aphansi noma ngisho nokushisa kwekamelo, kungani kunjalo? Ake sibheke isimo se-methane nomoya-mpilo. Isakhiwo samakhemikhali se-methane siqukethe izibopho ezine ezifanayo ze-carbon-hydrogen Singacabanga ngesibopho se-ch se-methane njengentwasahlobo eqinile ikakhulukazi. Le ntwasahlobo i-taut kakhulu futhi idinga amandla amaningi ukwelula. Kumakhemikhali, leli "Force" lingamandla adingekayo ukuphula isibopho se-CH. Leli namandla obugqi amandla okwenza izibopho ze-methane ch ezizinzile ngokuqinile futhi zinzima kakhulu ukudiliza noma ukusabela ngaphansi kwezimo ezijwayelekile. Ngakolunye uhlangothi, ukusabela kwamakhemikhali, amaqembu asebenzayo avame ukwenziwa ngaphansi kokusebenzisana kwe-polar (ukusebenzisana kwe-polar kuyinto molecule ebhekene ne-molecile eyodwa kahle, kanti nokwakheka kahle kwe-methane molecule kuvimbela Kusuka ekukhiqizeni ubumbano obunjalo (ngokusho kokuhlelwa kwamamolekyuli, i-molecule enendiza yokulinganisa ayinanhlekelele) futhi ayikwazi ukunikeza amaqembu asebenzayo. Ngakho-ke, ukusebenza nokuguqulwa kwe-methane kuyinselele enkulu futhi kuvame ukudinga izimo ezinzima njengamazinga aphezulu (600-1100 ° C) noma ezinye "ama-degment" afana nama-acid anamandla nama-radicals mahhala ukusiza kusebenze i-methane. Ngakho-ke, ubunzima obukhulu ekufezekiseni ukusebenza okushintshiwe okuphansi kwe-methane nomoya-mpilo kulele kanjani kusebenze isibopho se-ch methane, okungukuthi, ukuthi welula kanjani "intwasahlobo" ku-CH Bond. Isimangaliso se-Catalyst Ososayensi baqhamuka nesixazululo esihle sale nkinga, futhi bakhetha ukusebenzisa i-catalyst ukusiza ukwenza kusebenze i-methane emazingeni aphansi (i-catalyst kuyinto yamakhemikhali engaguquki ngaphambi noma ngemuva kokuphendula, kepha isheshise ukusabela ngokushintsha imali ephansi yamandla adinga ukufakwa ekuphendukelweni ukuze kwenzeke). Ngo-2023, iphephabhuku le-Nature Catalysis libika ngenqubo yokuthola ukuguqulwa okuqondile kwe-methane nge-C1 oxides (i-methanol (ChOOH), ne-HOCOOH), ne-HOCHOH), ne-Hochy2), ne-Hosy2) catalyst ngo-25 ° C. Ukuguqulwa kwe-Methane kwama-4.2% futhi cishe ama-oxygenates acishe abe ngu-100% Le mos2 i-mos2 ukuphela kokuphela okubikwa kuze kube manje lokho kungabona ukuguqulwa kokushisa kwekamelweni kwe-methane nomoya-mpilo. Konke lokhu kungenxa ye-geometry eyingqayizivele ne-elekthronikhi yesayithi le-MO emaphethelweni e-mos2. Lesi siza se-Mo Mo sinomsebenzi ophezulu wokusebenzisa i-oksijini endaweni enamanzi, ekwakheni imilingo O = MO = O * izinhlobo. Lolu hlobo lwenza i-carbon-hydrogen bond kulula ukuphula futhi yehlise amandla okusebenza kwebhondi ye-CH Methane, ngakho-ke kwandise kakhulu ukusebenza kabusha kwe-methane, futhi ngaleyo ndlela ukubona ukusebenza okuphansi kwe-methane nomoya-mpilo. Lokhu kutholwa kuzoletha amathuba amaningi okusetshenziswa kwamandla okuzayo kanye nokuvikelwa kwemvelo, kanye nokusinika ukuqonda okujulile kweqhaza elimangalisayo lama -catalysts nama-auxiliaries.
3.Izinqolo eziqashiwe ezinamandla zokusebenzisa okushisa okuphansi kwe-methane Ukubona ukuguqulwa okuqondile kwe-catalytic kwe-methane nomoya-mpilo emazingeni okushisa asekamelweni, futhi uguqule i-methane kugesi yemvelo kwamanye amakhemikhali awusizo, athuthukise kakhulu izinga lokusetshenziswa kwegesi yemvelo, anciphise imvelo, futhi avikele kangcono ukuthuthuka kwamandla . Okwesibili, njenge-greenhouse kagesi, i-methane ineminyaka yesibili kuphela ku-carbon dioxide emgaqweni wayo ekushiseni komhlaba. Uma i-methene ingaguqulwa ibe ezinye izinto, ingasisiza ukunciphisa ukuphuma kokungcoliswa komoya (isib. I-Carbon oxides, i-nitrogen oxides, ama-suldfocarbons, ama-hydrocarbon, kanye namakhompiyutha ethershise) futhi anciphise ingcindezi yokufudumala komhlaba.




